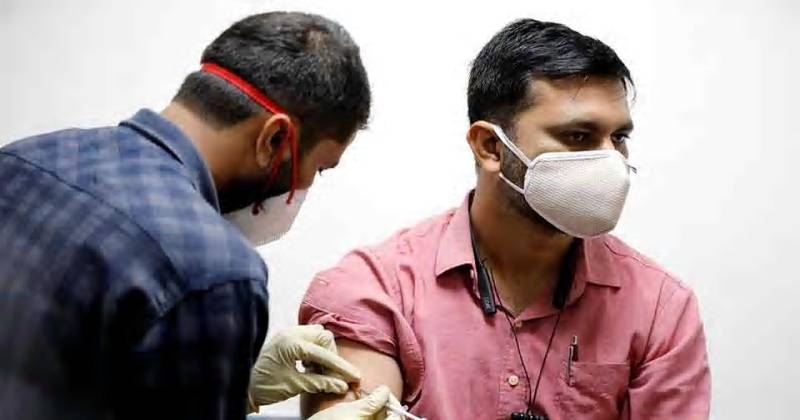
The regulatory experts said, “The recommendation by an advisory panel of the US Food and Drug Administration that the regulator provide emergency use approval for Pfizer Inc and BioNTech’s Covid-19 vaccine will strengthen their case when they seek similar authorization by Indian authorities.”
A senior official in the Central Drugs Standards Control Organization said, “This will definitely give them some added advantage if Pfizer-BioNTech vaccine candidate application is put before the subject expert committee here in India with the US FDA approval already in place.”
An official said, “It has not happened so far that the approval has been granted for sale and distribution without clinical trials on Indian population. The number of participants can remain comparatively smaller like between 10 and 40 but the results of tests conducted locally were sought. However, there is always a provision to waive local trials and go by the data generated globally in case of a pandemic situation when the processes have to be expedited. The committee is authorized to take a call on this.”

Post Your Comments