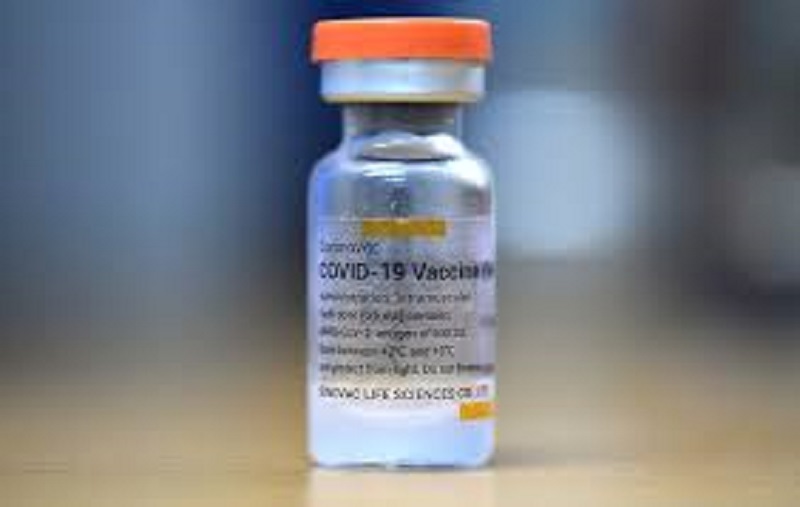
Geneva: The World Health Organization (WHO) has approved another Covid-19 vaccine for emergency use. The WHO has approved the Covid-19 vaccine manufactured by a Chinese company by Sinovac Biotech. It is the second Chinese vaccine to get approval from WHO.
The Sinovac vaccine is already in use in 22 territories around the world. Apart from China, the countries using Sinovac include Chile, Brazil, Indonesia, Mexico, Thailand, and Turkey.
Earlier, WHO has approved the Covid-19 vaccine made by another Chinese company, Sinopharm. Sinopharm is the first Chinese vaccine to be approved by the WHO. Till now WHO has approved vaccines made by Pfizer/BioNTech, Moderna, Johnson & Johnson, and the AstraZeneca vaccine being produced in India, South Korea, and the EU.
Also Read: Covid-19: Antibody cocktail therapy begins at Delhi’s Ganga Ram hospital
WHO emergency listing is a signal to national regulators on a product’s safety and efficacy. It would also allow the vaccine to be included in COVAX, the global programme to provide vaccines mainly for poor countries.

Post Your Comments