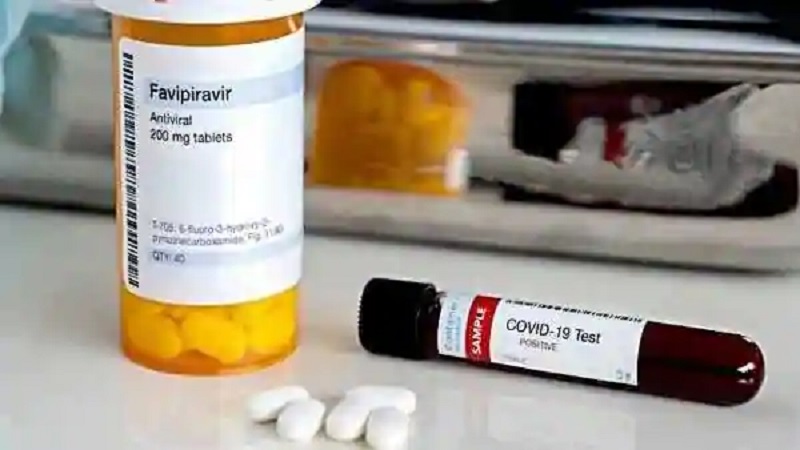
Drug manufacturing company, Glenmark Pharmaceuticals has launched the antiviral drug ‘Favipiravir’, under the brand name ‘FabiFlu’, for the treatment of patients with mild to moderate Covid-19. The medicine was released after getting manufacturing and marketing approval from the Drugs Controller General of India (DCGI).

The recommended dose of Favipiravir is 1,800 mg twice daily on day one, followed by 800 mg twice daily up to day 14. It is a prescription-based medicine. The drug is claimed to have an efficacy of over 80% in the treatment of COVID-19 mild to moderate patients.
FabiFlu is the first oral favipiravir-approved medication in India for the treatment of Covid-19. The medicine is priced at Rs 103 per tablet.The drug will be available as a 200 mg tablet at a maximum retail price of Rs 3,500 for a strip of 34 tablets.
Favipiravir is sold under the brand name ‘Avigan’ and is approved in Japan since 2014 in treating influenza. It is already being used in the therapeutic management of COVID-19 in Bangladesh and UAE.

It is under approval process in Egypt and Jordan and is a part of the treatment protocol in Russia, Japan and Saudi Arabia. About 18 global clinical trials in 3,000 subjects are going on including in India, USA, Canada, Italy, China, France, UK and other countries.

Post Your Comments