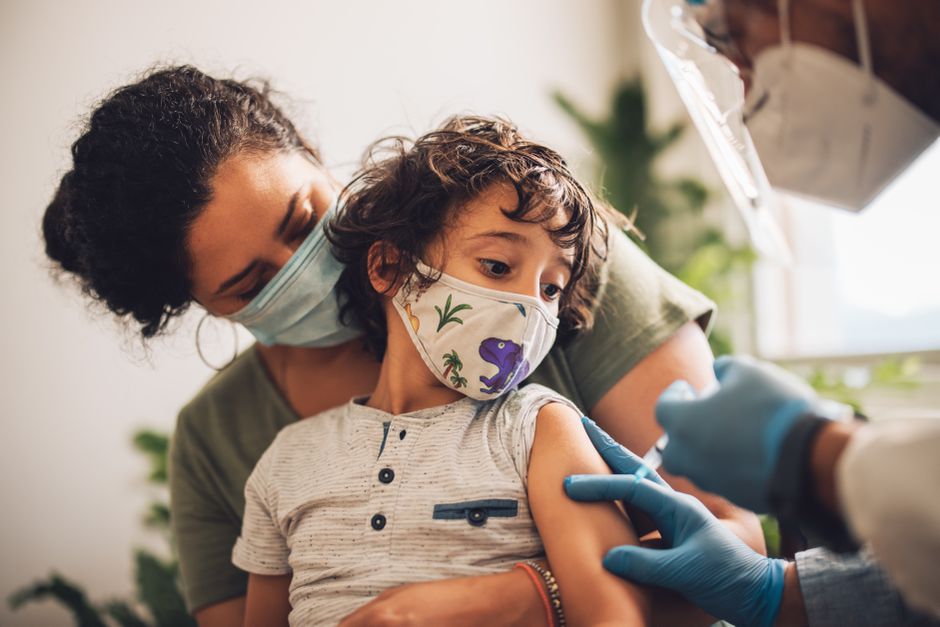
Parents in the Unites States, wanting to vaccinate their young children, were given a pleasant surprise earlier this month when it was revealed that the first COVID vaccine for children under the age of five could be approved by the end of February, months earlier than many experts thought.
That expedited approval could be the outcome of an innovative, potentially unprecedented, approach being used by the Food and Drug Administration in deciding whether to provide emergency use authorisation for Pfizer’s low-dose vaccination for young children.
The approximately 23 million children under the age of five are the last group of Americans who do not have access to immunizations. The urgency of protecting older populations who are at greater risk has been a major reason for this, but research has also been hindered by the specific challenges of testing medicines on young children and unsatisfactory clinical trial outcomes. Pfizer revealed mixed findings from the two-dose regimen it had been studying in small children in December. Children aged 6 months to 2 years had an antibody response equivalent to that of vaccinated adults, whereas children aged 2 to 4 years did not. Because Pfizer data has not been made public, it is unknown how far below the target that was.
Pfizer is currently testing a third shot in the aim of eliciting a more strong reaction in all young children. Under normal circumstances, the FDA would wait for the completion of that experiment before considering approval of a three-dose regimen. However, the agency is currently debating whether to authorise only the first two shots right away in the hope that the third dose will be approved later.
Health experts are divided on whether it is prudent for the FDA to approve a two-dose plan for children without waiting for data on the third dose.
The key advantage of the technique, according to supporters, is speed. They suggest that getting the first two injections into children’s arms now will provide some protection and a head start toward full immunity once the third one becomes available. They also argue that, while children are less prone to contract severe diseases, getting them vaccinated as soon as possible is still critical.
More than 280 children aged 4 and under have died from COVID since the outbreak began, and child hospitalizations have reached new highs during the Omicron wave. Experts think that, despite the vaccine’s minimal dangers, the potential advantages — which include relief for worried parents and fewer COVID-related childcare delays — make hastening the licencing process desirable.
Critics, on the other hand, are concerned about the hazards of licencing a vaccine without convincing evidence that it would deliver the required level of immunity. They suggest that it would be best to wait until there is comprehensive data on all three doses, which could be available within the next month or two. Many people are concerned that authorising the two-dose regimen will increase vaccine hesitation among parents, rather than the potential wasted effort of putting out an ineffective vaccine. Vaccination rates among older children have plateaued, and polls show that the majority of parents with young children are doubtful about the necessity to vaccinate them. Critics of the FDA’s plan are concerned that news about the approval of an experimental vaccine will exacerbate the problem.

Post Your Comments