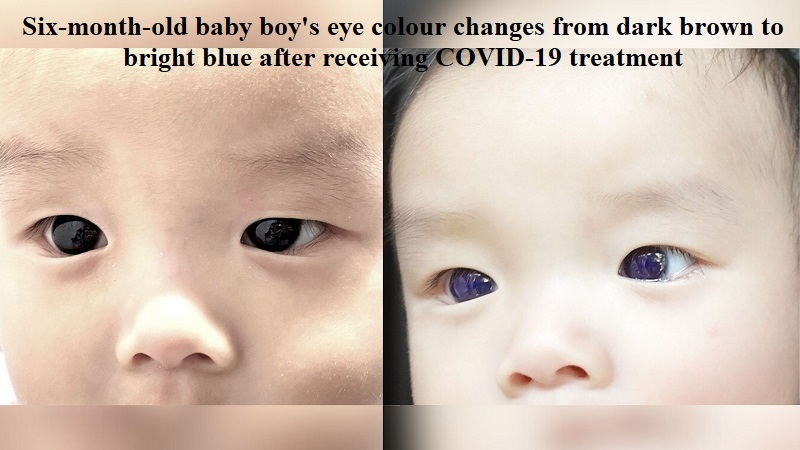
Following a straightforward COVID-19 treatment, there are reports of a six-month-old baby boy experiencing a change in eye color from dark brown to vibrant blue.
The infant, hailing from Thailand, was diagnosed with COVID-19 after displaying symptoms of fever and cough for a day, as documented in a case featured in the medical journal “Frontiers in Pediatrics.”
The attending physician reportedly prescribed favipiravir, an antiviral treatment approved by the Thai Ministry of Public Health for children with mild to moderate COVID-19 symptoms. However, 18 hours after initiating the treatment, the baby’s mother observed a change in his eye color. Worried, she contacted medical professionals who advised her to discontinue the treatment immediately, as reported by the New York Post.
The discoloration gradually faded after five days, and the baby boy’s eyes returned to their original color. Medical examinations also revealed that the infant’s cornea had cleared, and the bluish tint had disappeared from the surface of the iris or the anterior lens capsule.
Although the exact cause of the discoloration remains unknown, experts suspect it may be related to the fluorescence (emission of absorbed light) resulting from the drug’s metabolites or additional tablet components like titanium dioxide and yellow ferric oxide. Some studies have indicated a potential correlation between the concentration of favipiravir and fluorescence intensity, particularly in human hair and nails.
Notably, the child did not experience any vision impairment and successfully recovered from his COVID-19 symptoms.
Common side effects of favipiravir include mild hyperuricemia (elevated uric acid levels), diarrhea, and neutropenia (reduced white blood cell neutrophils), accounting for approximately 20 percent of adverse reactions.
This case isn’t the first instance of corneal discoloration associated with favipiravir use in COVID-19 treatment. In 2021, a 20-year-old Indian man reported a similar effect. He noticed that his dark brown eyes had turned bright blue on the second day of taking favipiravir. Doctors advised discontinuation of the medication, and his eye color returned to normal within one day.
While several countries, including Japan, Russia, Ukraine, Uzbekistan, Moldova, and Kazakhstan, have approved favipiravir for COVID-19 treatment, it has not received FDA authorization. It was granted emergency use approval in Italy in 2020.
Numerous U.S. researchers suggest the drug’s effectiveness in COVID-19 treatment, as several studies have evaluated its capacity to combat the disease. Nevertheless, the Food and Drug Administration has yet to grant formal approval for favipiravir.

Post Your Comments