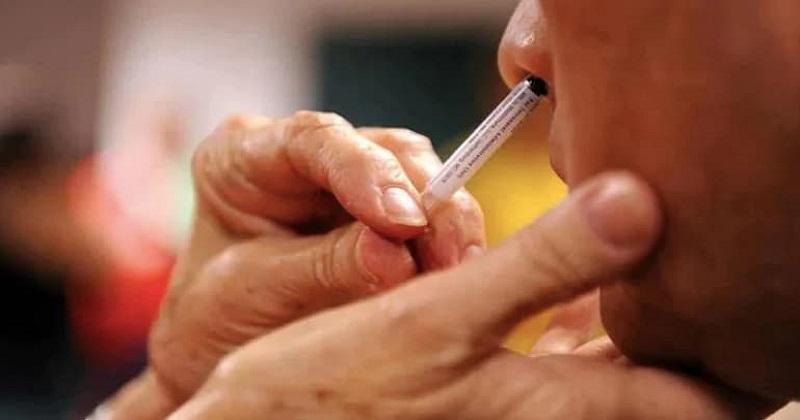
Hyderabad: Bharat Biotech, which developed the first nasal vaccine against Covid-19, has received the regulator’s nod for conducting Phase 2 and 3 clinical trials, the Department of Biotechnology said on Friday.
‘Bharat Biotech’s intranasal vaccine is the first nasal vaccine that has received the regulatory approval for Phase 2/3 trials,’ the Department of Biotechnology said.
Read also: UP: CM reviews situation in flood-affected districts
In India, this is the first Covid-19 jab to undergo human clinical trials. Phase 1 clinical trials have been completed in age groups ranging from 18 to 60 years. The company spokesperson said the vaccine administered to healthy volunteers in Phase I clinical trial was well tolerated and no serious adverse events were reported.
In preclinical toxicity studies, the intranasal vaccine was deemed safe, immunogenic and well-tolerated, and could elicit high neutralizing antibody levels in animal studies.
According to Dr. Renu Swarup, Secretary, Department of Biotechnology, ‘The Department through Mission Covid Suraksha is committed to the development of safe and efficacious Covid-19 vaccines. Bharat Biotech’s BBV154 Covid vaccine is the first intranasal vaccine being developed in the country entering into late-stage clinical trials.’

Post Your Comments